



Diabetes has become a major problem in India, impacting a staggering 101 million individuals, often compounded by obesity. Despite the various treatments available, many diabetes patients struggle to manage their blood sugar levels effectively. Clinical guidelines emphasise the importance of a balanced diet and lifestyle interventions for type 2 diabetes management. However, India faces unique challenges like the consumption of high-fat, high-carb diets. These factors contribute to poor diabetes control, further complicated by a lack of awareness about the role of diet in managing diabetes.
To address these challenges, a vital approach is to prioritise a balanced, calorie-controlled dietary intervention. One effective strategy is incorporating diabetes-specific nutritional supplements (DSNS). These supplements can be used to replace one or two meals with meal replacement products, providing essential nutrients. This approach can help individuals with type 2 diabetes overcome meal planning difficulties and manage their blood sugar levels effectively.
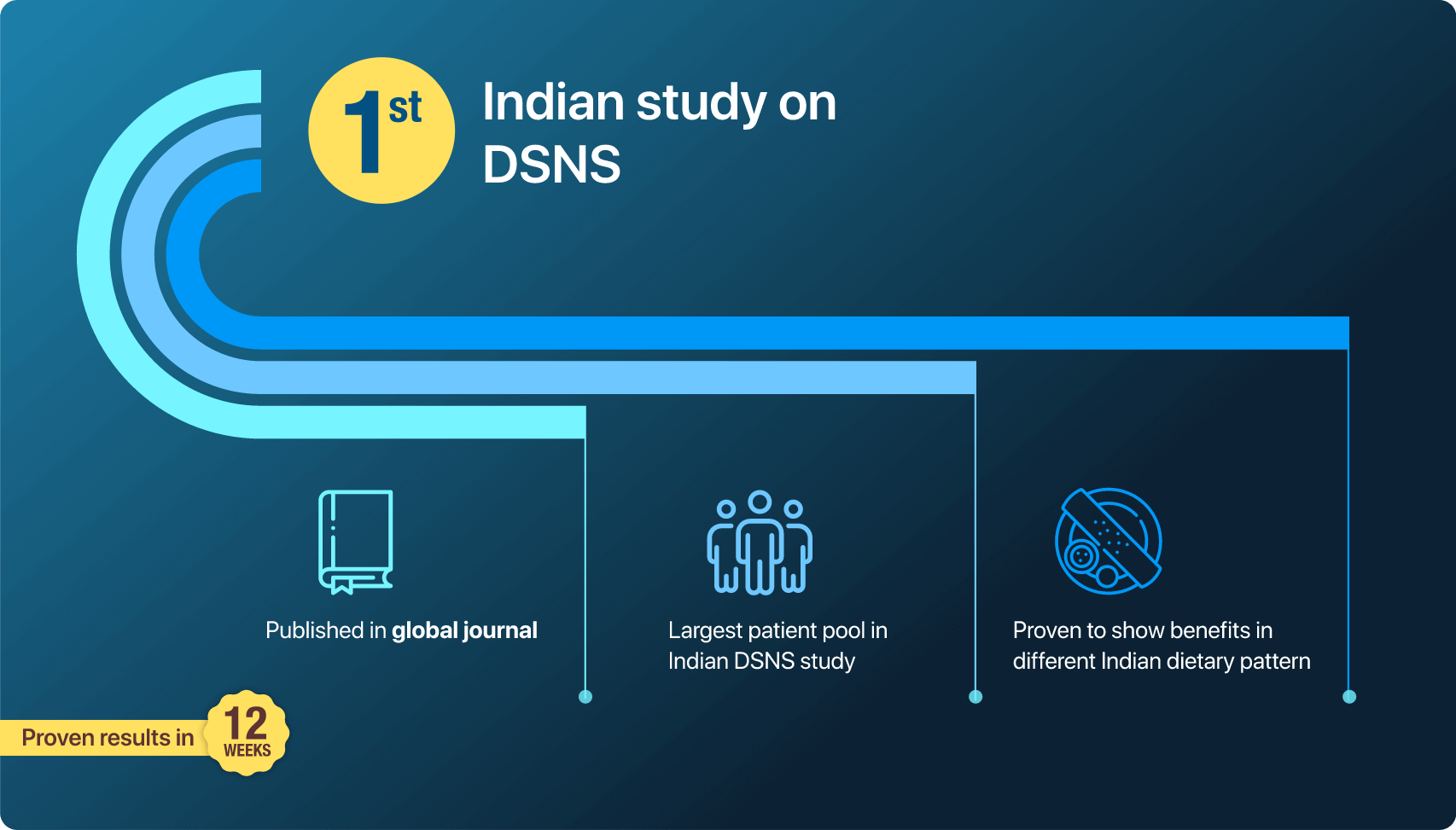
Recent research conducted in India, known as the PRIDE study (Partial Meal Replacement on Blood Sugar Levels and Body Weight in Indian Patients with Type 2 Diabetes), throws light on the effectiveness of this approach. It is first one-of-its-kind Indian study on diabetes nutrition supplement, involving the largest patient pool in Indian DSNS study. The 12-week study included 171 participants.
Received Prohance-D (diabetic specific nutrition supplement) as a dietary intervention alongside standard care (i.e., diabetes treatment along with dietary counselling).
Received only standard care (SOC group).
The primary objective of the PRIDE study was to explore the potential benefits of Prohance-D for type 2 diabetes patients by replacing one meal with Prohance-D (50 g) alongside standard diabetes treatment. The study assessed various factors such as blood sugar levels (HbA1c), blood sugar profiles, waist circumference, body weight, lipid profile, and factors impacting the participants’ quality of life.
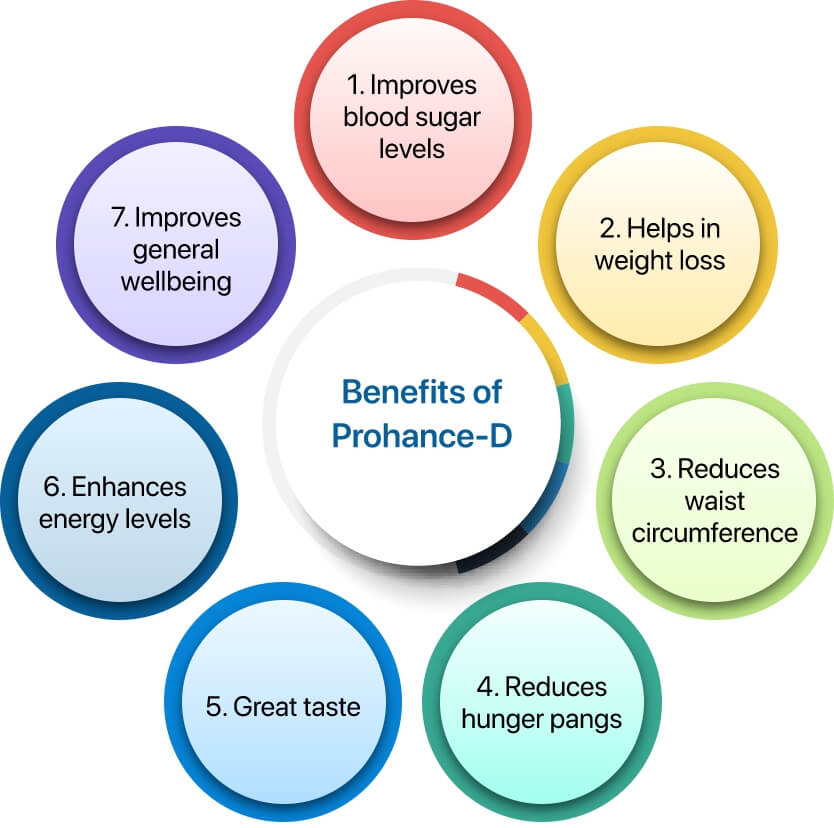
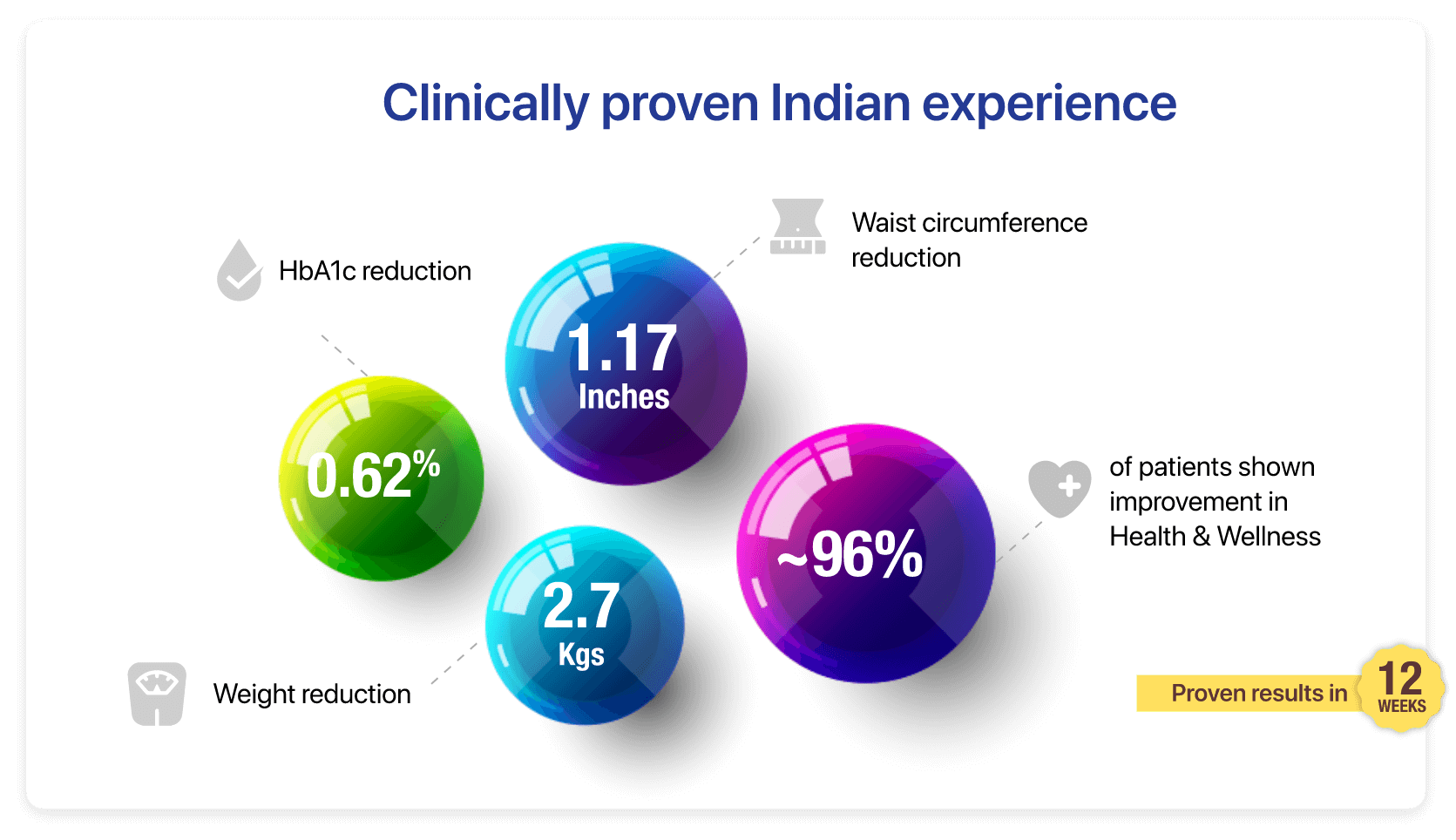
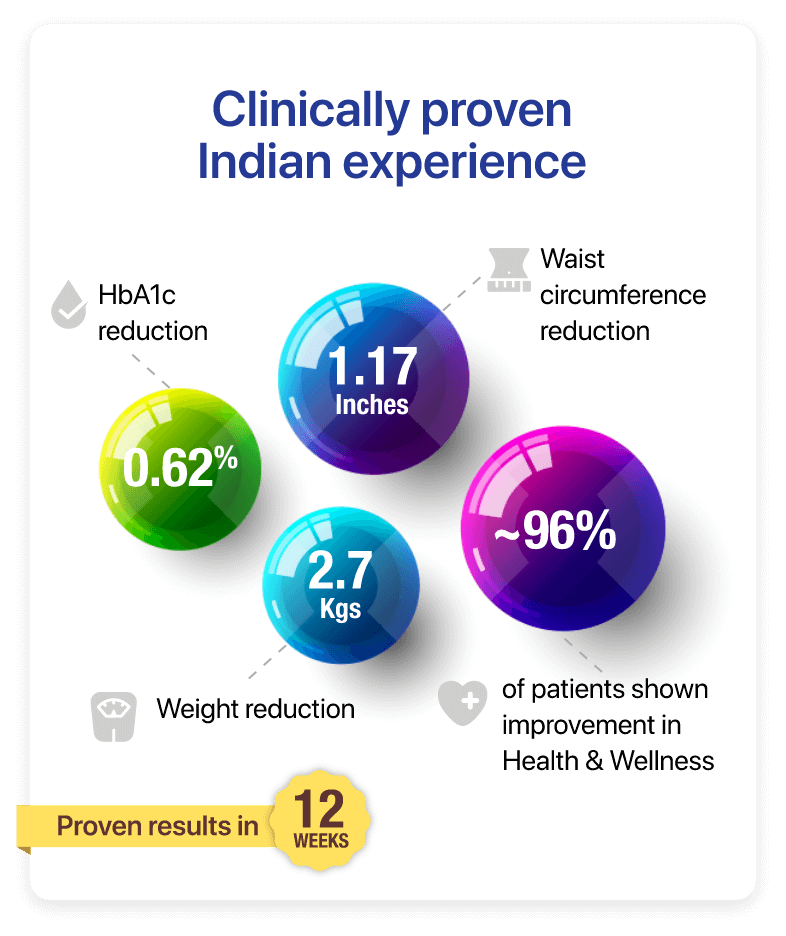
The PRIDE study showed that the group using Prohance-D experienced a notable 0.62% reduction in HbA1c levels compared to the control group at the end of 12 weeks.
In PRIDE study, the participants using Prohance-D showed a remarkable mean weight loss of 2.70 kg and a reduction of 1.17 inches of waist circumference at the end of 12 weeks.
In the PRIDE study, the group using Prohance-D demonstrated greater reductions in both fasting (15.58 mg/dL) and postprandial sugar levels (23.64 mg/dL) than the control group.
PRIDE study showed that 55.3% of patients did not feel hungry after taking Prohance-D as compared to 44.8% patients in SOC group. Thus, Prohance-D helps to reduce hunger pangs by increasing the satiety level.
Patients taking Prohance-D never felt lethargic (64.5%) and showed improvement in general well-being (73.7%) as compared to SOC. This indicates that Prohance-D helps to enhance energy levels and improve the over well-being.
96% patients were satisfied with the taste of Prohance-D which can be a prime indicator of improving adherence to the partial meal replacement in diabetes management.
DSNS such as Prohance-D has the capability to play a vital role in the management of diabetes and PRIDE study was a landmark step in this direction to underscore the benefits of Prohance-D in diabetes. The study revealed a plethora of advantages including effective blood sugar level control, weight management, and improved overall health outcomes. As we continue to explore innovative strategies for diabetes management, Prohance-D stands out as a promising tool for people with diabetes to help them take control of their health and well-being.